Many of us take comfort in the fact that it’s often not quantity, but quality that really matters. Well, it turns out this isn’t the case for using AAV. When it comes to infecting cells, titer, the amount of virus used, really does matter. (*psst*, quality definitely also matters).
Check out this post for a refresher on AAV titers
Making contact with a cell
Although an individual AAV particle’s ability to infect a cell is dependent on its capsid construction and the cell it’s infecting, getting these particles to infect cells is also very titer-dependent. In order for an AAV particle to infect a cell, it has to come into physical contact with that cell. Since there is a limited amount of surface area on each cell, the way to get more AAV in contact with the cell is to increase the AAV concentration.
In this flowchart, you can see the dwindling proportion of AAV particles that make it through each part of the cell on their way to the nucleus where their gene cargoes can be expressed.
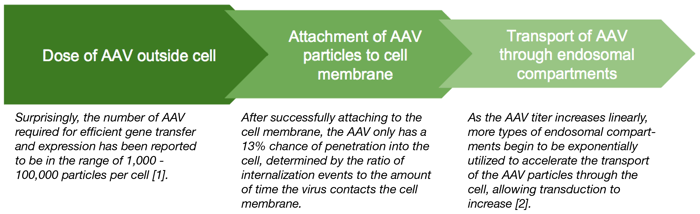
The bottom line is that a lot of AAV particles are needed to get a few good ones into a cell to produce expression. The higher the titer, the better.
AAV transport and transgene expression
 It’s clear that we need high titers, as contact between the cell and any individual AAV particle is rare. But simply getting into the cell may not always be enough and therefore higher titer will be required. For example, retrograde AAV gets transported up the cell’s axon. While the mechanism for this function is not yet fully understood, we can imagine that this transport is probably not 100% efficient. So the few AAV particles that have entered the cell now have to travel up the axon, through the cell, and potentially spread out over an even larger area, thus necessitating even higher retrograde AAV titers for successful transduction.
It’s clear that we need high titers, as contact between the cell and any individual AAV particle is rare. But simply getting into the cell may not always be enough and therefore higher titer will be required. For example, retrograde AAV gets transported up the cell’s axon. While the mechanism for this function is not yet fully understood, we can imagine that this transport is probably not 100% efficient. So the few AAV particles that have entered the cell now have to travel up the axon, through the cell, and potentially spread out over an even larger area, thus necessitating even higher retrograde AAV titers for successful transduction.
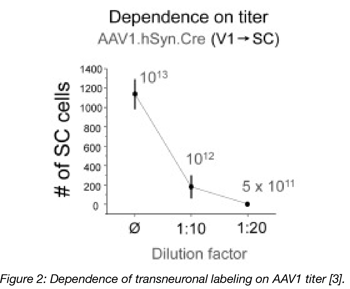
In another example, neuronal gene expression by an anterograde AAV was shown to drop off drastically with lower titers. As you can see in figure 2, the expression from the 5x1011 titer was essentially nonexistent, as there wasn’t enough virus to produce any observable expression. Doubling this titer to 1012 resulted in expression in roughly 200 cells. Increase the titer to 1013 resulted in a roughly 6-fold increase in expression [3, Figure 2]. Note how expression increases exponentially with an increase in titer. That means that once you drop below a certain (very high) threshold of viral particles per cell, you may not be able to detect AAV-mediated gene expression in a target cell.
Practical considerations
If you have high quality AAV, you can address the issue of low titer by concentrating your virus. See our Concentrating AAV Protocol (Step 10) for instructions on how to do this. Concentrating your virus will allow you to add many more viral particles to your solution while still using a volume that’s compatible with your experimental setup.
Some experiments require higher gene expression and therefore higher titer than others. High titers are often necessary to visualize expression of fluorescent proteins, for example, as many viral particles must infect a cell to achieve visible expression of the protein. However, expression of receptors may not need to be as high to achieve an observable signal in your experiment, therefore titer may not need to be as high when delivering receptor constructs with AAV. In the case of channelrhodopsins, fewer channel proteins are required for the transduced cell to become light responsive than fluorescent proteins required to get a good fluorescent signal. It may not be necessary to concentrate this type of vector to such a high titer when looking for effective gene expression.
How do you achieve high titers in the first place?
Some AAVs are known to be difficult to produce in high titer. These include AAV2, which is known to adhere to the cell interior, causing less particles to be released into suspension and lowering the number of viral particles that can be harvested from the surrounding media.
Nonetheless, there are a variety of ways you can optimize AAV production. We’ve listed some simple steps you can take below but you should read our AAV Production Protocol for additional best practices.
- Keep your cells happy - Make sure your cells are as healthy as can be to prepare them for the arduous process of generating viral particles.
- Limit tube transfer - the fewer opportunities your viral particles have to stick to your tubes, the higher titer you will have. When possible, use low-bind or siliconized tubes to store samples as it is more difficult for viral particles to adhere to these tubes.
- Rinse off the filter - When using a concentration filter column, make sure to rinse off the surface of the filter membrane with the surrounding solution to ensure excess viral particles do not remain adhered to the membrane.
- Be careful when diluting - if you end up concentrating your AAV above your desired titer and want to dilute it, err on the side of caution when adding buffer back to the solution. Add a bit less buffer than you would to achieve the exact desired concentration to avoid over-diluting, and make sure to remeasure your titer after diluting to confirm.
 Luke Makana Hanley is a Research Team Lab Tech at Addgene working on methods to improve AAV production.
Luke Makana Hanley is a Research Team Lab Tech at Addgene working on methods to improve AAV production.
References
1.Clark, K. Reed. "Recent advances in recombinant adeno-associated virus vector production." Kidney international 61.1 (2002): S9-S15. PubMed PMID: 11841606.
2. Schultz BR, Chamberlain JS. Recombinant Adeno-associated Virus Transduction and Integration. Molecular therapy : the journal of the American Society of Gene Therapy. 2008;16(7):1189-1199. doi:10.1038/mt.2008.103. PubMed PMID: 18500252. PubMed Central PMCID: PMC2574934.
3. Zingg, Brian, et al. "AAV-mediated anterograde transsynaptic tagging: mapping corticocollicular input-defined neural pathways for defense behaviors." Neuron 93.1 (2017): 33-47. PubMed PMID: 27989459. PubMed Central PMCID: PMC5538794.
Additional Resources on the Addgene Blog
- AAV Titers: Where do they come from and what do they mean?
- AAV Vector Quality Control: Going the Extra Mile with NGS
- AAV: Versatile Viral Tool for Gene Expression in Mammals
Additional Resources on Addgene.org
Topics: Viral Vectors, Viral Vectors 101, Viral Vector Protocols and Tips, AAV






Leave a Comment