We’re always looking for ways to improve our shipment processes. After reading a publication describing how short term storage on dry ice can shift sample pH, we wondered whether or not the dry ice we use to keep viruses frozen during shipment was having an impact on the samples. We therefore devised a few experiments to determine if our tubes were permeable to the CO2 released from dry ice, and whether this affected the pH of our viral samples. Read on to learn how aqueous samples might be affected by dry ice, and specifically how dry ice can affect virus from Addgene.
Bottom line: there’s good news and there’s bad news. The bad news is that some of tubes’ o-rings are, in fact, permeable to CO2 at low temperatures (-80°C) and once in the tube, the CO2 can alter the pH of the liquid sample. The good news is that this effect is reversible and the pH shift can be prevented. Keep this information in mind if you’re planning on shipping something on dry ice or if you’re receiving samples on dry ice - it may prevent you from seeing some unexpected results.
Order Ready to Use Viral Preps from Addgene
Measuring the pH of our samples: What we did
To measure the pH of our samples, we used a solution of cell culture medium, which is buffered and contains the pH indicator phenol red. We froze samples in a -80°C freezer and then moved them to a closed dry ice storage container for 48 hours (to replicate how our viruses are shipped), where they would be exposed to a closed environment containing CO2 (from the dry ice). After 48 hours of CO2/dry ice storage, we then thawed the samples at room temperature and used the color of the liquid solution and pH paper to measure the pH. We compared the dry ice-stored samples to controls that were only stored in a -80°C freezer and not exposed to CO2/dry ice. All samples were tested in duplicate.
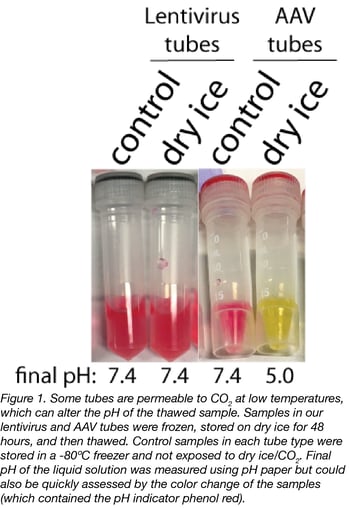
What we found
Not all tubes (or rather, not all O-rings) were permeable to CO2 at low temperatures. We tested two different types of tubes: the ones we use to store lentivirus and the ones we use to store AAV. Our results showed that, once thawed, there was no effect on the pH of the samples stored in our lentivirus tubes (Fig. 1). However, once thawed, there was a substantial reduction (from 7.4 to 5.0) in the pH of samples stored in our AAV tubes (Fig. 1).
It’s important to note that the change in pH only occurred when the solution thawed, as the CO2 was confined to the tube headspace when the samples were frozen. This was clear by the gradual change in pH visible at the sample interface upon thaw (Fig. 2). Before thawing, all the samples were at the control pH of 7.4 as indicated by color (data not shown).
What we did next: Venting the tubes
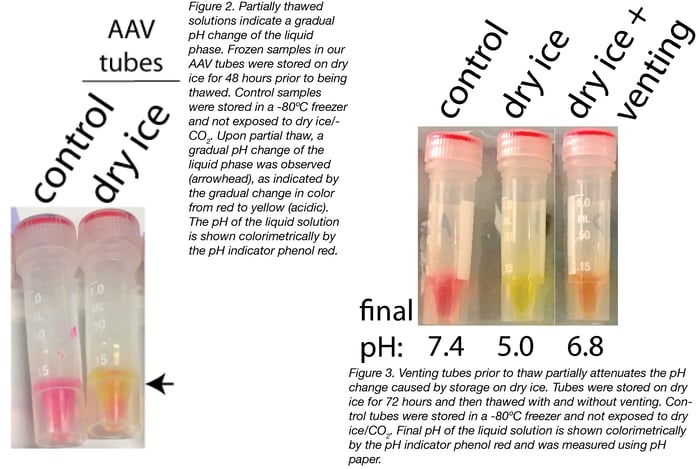
We then tested whether venting the tubes prior to thaw released the CO2 from the headspace, thus mitigating the pH effects of dry ice storage. To do this, we stored samples on dry ice for 72 hours and then vented the tubes by completely removing the caps, incubating the tubes in an upright position for 30 seconds, and then recapping the tubes prior to thaw. We compared vented and non-vented tubes to controls that were not exposed to dry ice.
Does venting help?
We found that 30 seconds of venting attenuated the pH change caused by storage on dry ice (Fig. 3). However, 30 seconds was not sufficient to prevent any pH change, as we still observed a slight change in pH (from 7.4 to 6.8). It’s possible that a longer vent time would be sufficient to return the sample back to the original pH.
What about putting it back in the freezer?
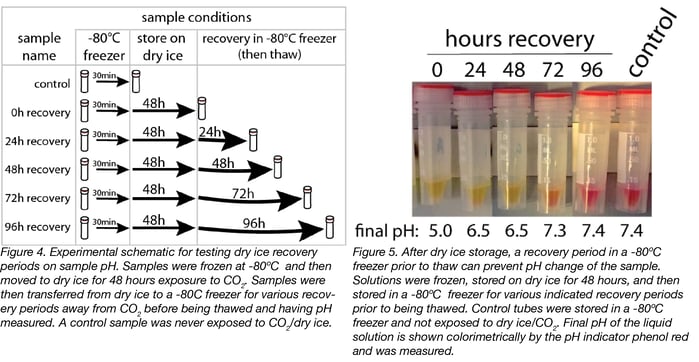
We then tested whether returning the tubes to the -80°C freezer after storage on dry ice allowed the CO2 to leave the tubes, and would prevent the pH change upon thaw (Fig.4). To test this, we froze the tubes at -80°C and then moved them to closed storage on dry ice for 48 hours. We then moved the tubes back to a -80°C freezer for various recovery periods between 0 and 96 hours. We compared these samples to controls that were only stored at -80°C. We then thawed the tubes after the indicated incubation periods, and measured the pH.
We found that tubes subjected to a recovery period of at least 96 hours had the same pH as control tubes that were never stored on dry ice/exposed to CO2 (Fig. 5). Basically, the CO2 goes in and the CO2 goes out.
 Conclusions and handling recommendations
Conclusions and handling recommendations
Not all O-rings function optimally at -80°C. For samples that are shipped on dry ice, this can affect the pH of the sample, which may have some effect on the contents of the sample. For example, one study found that dry ice storage affected the performance of antibodies, because of effects of pH on protein stability (Murphy et al., 2013). However, the pH changes in our solutions are transient and return to normal once the tube is opened and equilibrium with ambient air is restored. We have no evidence to suggest that the transient change in pH affects AAV function. Furthermore, another study tested AAV infectivity after a range of pH exposures. They showed that AAV infectivity was not altered by a short 2-hour exposure to pH as low as 2.5 (Potter et al., 2014). They also showed that even 24 hours of exposure to pH 5.1 did not alter the infectivity of the virus. However, there was a substantial reduction in infectivity after 3 weeks of exposure to pH 5.1 (Potter et al., 2014).
Based on our results and the results reported elsewhere, we recommend that once you receive your AAV from Addgene, you store the AAV at -80°C for 96 hours prior to thaw. However, if this is not possible, we recommend both venting the tube for at least 30 seconds prior to thaw, and also using the thawed virus within 24 hours.
References
1. Murphy BM, Swarts S, Mueller BM, van der Geer P, Manning MC, Fitchmun MI. Protein instability following transport or storage on dry ice. Nat Methods. 2013 Apr;10(4):278-9. PubMed PMID: 23538862.
2. Potter M, Lins B, Mietzsch M, Heilbronn R, Van Vliet K, Chipman P, Agbandje-McKenna M, Cleaver BD, Clément N, Byrne BJ, Zolotukhin S. A simplified purification protocol for recombinant adeno-associated virus vectors. Mol Ther Methods Clin Dev. 2014 Aug 13;1:14034. PubMed PMID: 26015974. PubMed Central PMCID: PMC4420252
Additional Resources on the Addgene Blog
- Learn How You Can Use the Viral Service
- Tips for 1st Time CRIPSR User (By a 1st Time CRISPR User)
- Tips for Titering Your Lentivirus Preps
Additional Resources on Addgene.org
- Check out the Viral Service
- Find Virus Protocols
- Browse Our Viral Vectors
Topics: Viral Vectors, Addgene’s Viral Service






Leave a Comment