 Originally published Sep 30, 2014 and last updated Dec 10, 2020 by Benoit Giquel.
Originally published Sep 30, 2014 and last updated Dec 10, 2020 by Benoit Giquel.Delivering gRNA and Cas9 using adenoviral vectors
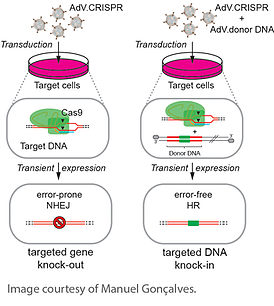
As compared to other viral vectors, AdVs can provide a large transgene packaging capacity. For instance, with a packaging capacity of about 6 kb, the 1st generation of AdVs have already a packaging capacity larger than AAV vectors. This capacity is large enough to carry the Cas9 gene and a gRNA expression cassette in one single viral particle. The earliest explorations of the use of AdV for in vivo CRISPR-Cas delivery utilized the Non Homologous End Joining (NHEJ) DNA repair mechanism to knock out genes as a result of an insertion, a deletion or a frameshift (Ding et al., 2014, Wang et al., 2015). In these studies, the strategy employed was to deliver a standard AdV vector to the targeted organs or tissues with Cas9 and a gRNA designed to target a mutated gene. In a Scientific Reports paper introducing the delivery method in 2014, Manuel Goncalves lab report that AdV-mediated transduction of gRNA:Cas9 ribonucleoprotein complexes into transformed and non-transformed cells yielded rates of targeted mutagenesis similar to those achieved by isogenic AdVs encoding TALENs targeting the same chromosomal region. The CRISPR/Cas9-derived RNA-guided nuclease-induced gene disruption frequencies in the various cell types ranged from 18% to 65% (Maggio et al., 2014).
An alternative approach to NHEJ is to utilize the Homology Directed Repair (HDR) to knock in corrective genes. For this you would need to provide a DNA template to repair the double strand break via homology directed repair and you would probably need to use a two viral vectors system: one vector for Cas9/sgRNA and one vector for your specific DNA template. Using this two vectors system, Manuel Gonçalves' lab found in a second paper published in Nature Methods the same year that delivering RNA-guided nucleases or TALENs together with AdV donor DNA leads to a vast majority of AdV-modified human cells being subjected to scarless homology-directed genome editing (Maggio et al., 2014). Gonçalves said they attribute this phenomenon to the presence of terminal proteins capping the ends of linear double-stranded AdV genomes. Such protein-DNA structures presumably reduce the likelihood that donor DNA will interact with sporadic double-stranded chromosomal DNA breaks “that always happen naturally.”
These adenoviral CRISPR/Cas9 genome editing tools developed and demonstrated by Manuel Gonçalves and his colleagues at Leiden University Medical Center are now available at Addgene along with a description of their experimental protocol. The three plasmids which have been deposited to Addgene are: pAdSh.PGK.Cas9, pAdSh.U6.gRNAS1, and pAdSh.U6.gRNAGFP.
Gonçalves says that advantages of AdVs include their episomal nature and very efficient introduction of DNA into therapeutically relevant, non-transformed mammalian cells. These viral vector systems also work equally well in dividing and quiescent, post-mitotic mammalian cells.
Applications of CRISPR delivery by adenoviral vectors
Since then, other groups have successfully developed and used AdV vectors to edit genes in several biological systems. It has been used for instance in inherited disorders such as B-thalassemia, Sickle Cells Disease, Duchenne Muscular Dystrophy, Alpha1-antitrypsin deficiency (most widely used so far) or for cancer gene therapy such as nonalcoholic steatohepatitis cancer. In deficiencies of plasma proteins where Adv vector delivery is often used, the CRISPR approach based on HDR has the advantage of being a potentially universal treatment (Stephens et al., 2019). If sufficient gene transfer and knock-in is achieved, producer cells could produce corrective amounts of protein for any such disease. This technology therefore represents a potential platform that could be realized via simple switching of the relevant gRNAs and donor DNA
Adenoviral CRISPR/Cas9 genome editing tools can also be used to deliver Cas9 and DNA templates in somatic cells of adult animals in order to create animal models for particular cancer. That’s exactly what the lab of Andrea Ventura at the Memorial Sloan Kettering Cancer Center did to create a model of Eml4–Alk-driven lung cancer (Maddalo et al., 2014). These tools are available at Addgene and could be useful to labs wishing to create this kind of animal models.
Start using adenoviral vectors with your CRISPR/Cas9 research!
To find more information about the adenoviral delivery of CRISPRS/Cas9 using the Gonçalves and Ventura labs’ plasmids, including protocols, check out the plasmids at Addgene: pAdSh.PGK.Cas9 (expresses S. pyogenes Cas9 from the PGK promoter) and U6 promoter-driven guide RNA constructs, pAdSh.U6.gRNAS1 and pAdSh.U6.gRNAGFP, and also Adeno Cas9 and Adeno EA. Or if you're looking for a broader range of CRISPRs plasmid tools, find more plasmids, CRISPR technology guides, FAQs, and CRISPR resources on Addgene's CRISPR page.
References
Beatty MS, Curiel DT (2012) Adenovirus Strategies for Tissue-Specific Targeting. In: Applications of viruses for cancer therapy. Elsevier, pp 39–67
Ding Q, Strong A, Patel KM, Ng S-L, Gosis BS, Regan SN, Cowan CA, Rader DJ, Musunuru K (2014) Permanent Alteration of PCSK9 With In Vivo CRISPR-Cas9 Genome Editing. Circ Res 115:488–492 . https://doi.org/10.1161/circresaha.115.304351
Holkers M, Maggio I, Henriques SFD, Janssen JM, Cathomen T, Gonçalves MAFV (2014) Adenoviral vector DNA for accurate genome editing with engineered nucleases. Nat Methods 11:1051–1057 . https://doi.org/10.1038/nmeth.3075
Lukashev AN, Zamyatnin AA Jr (2016) Viral vectors for gene therapy: Current state and clinical perspectives. Biochemistry Moscow 81:700–708 . https://doi.org/10.1134/s0006297916070063
Maddalo D, Manchado E, Concepcion CP, Bonetti C, Vidigal JA, Han Y-C, Ogrodowski P, Crippa A, Rekhtman N, de Stanchina E, Lowe SW, Ventura A (2014) In vivo engineering of oncogenic chromosomal rearrangements with the CRISPR/Cas9 system. Nature 516:423–427 . https://doi.org/10.1038/nature13902
Maggio I, Holkers M, Liu J, Janssen JM, Chen X, Gonçalves MAFV (2014) Adenoviral vector delivery of RNA-guided CRISPR/Cas9 nuclease complexes induces targeted mutagenesis in a diverse array of human cells. Sci Rep 4: . https://doi.org/10.1038/srep05105
Stephens CJ, Lauron EJ, Kashentseva E, Lu ZH, Yokoyama WM, Curiel DT (2019) Long-term correction of hemophilia B using adenoviral delivery of CRISPR/Cas9. Journal of Controlled Release 298:128–141 . https://doi.org/10.1016/j.jconrel.2019.02.009
Wang D, Mou H, Li S, Li Y, Hough S, Tran K, Li J, Yin H, Anderson DG, Sontheimer EJ, Weng Z, Gao G, Xue W (2015) Adenovirus-Mediated Somatic Genome Editing of Pten by CRISPR/Cas9 in Mouse Liver in Spite of Cas9-Specific Immune Responses. Human Gene Therapy 26:432–442 . https://doi.org/10.1089/hum.2015.087
Additional resources on the Addgene blog
- How to Design Your gRNA for CRISPR Genome Editing by John Doench
- CRISPR-Cas9: Tips for Optimizing sgRNA Activity by John Doench
- All CRISPR Posts
Topics: CRISPR, CRISPR Expression Systems and Delivery Methods, Adenoviral Vectors







Leave a Comment