Understanding the biology behind brain development, learning, and behavior poses many challenges in neuroscience with many brain regions, neuronal networks, and specific neuronal populations to consider. To tease apart how different neurons impact a certain output, neuroscientists have begun to use more precise genetic tools: activators, inhibitors, sensors, recombinases, and more. With the help of adeno-associated viral vectors (AAV), neuroscientists can combine classical genetic tools with the manipulation of specific neuronal subtypes and determine how these changes affect the phenotype. Viral vectors and genetic approaches are continuously refined and developed so there are many combinations to choose from for your experiments!
Late last year, we hosted our first AAV Education and Development workshop aimed at brainstorming strategies for using combinations of AAV-based molecular tools for targeted neuronal manipulation. The goal of the workshop was to share the results of these discussions as a publicly accessible practical guide which was recently published in Frontiers in Neuroanatomy.
Here are the three main takeaways from the paper for designing experiments with AAV:
1. Getting your viral vector into your cell of interest: AAV tropism and route of administration
AAV tropism is defined by the viral capsid proteins that determine the AAV serotype. During transduction, the viral capsid proteins bind to the target cell’s surface proteins and this interaction determines the specificity and efficiency of the AAV serotype used for a specific cell type. Find some transduction characteristics of select AAV serotypes in Table 2 from the paper.
Delivery of AAV into the brain can usually be achieved by a directed brain injection, but some serotypes can cross the blood brain barrier after intravenous injection. Even when injected into the same place in the brain, certain serotypes spread further from the injection site. For an overview of AAV administration routes for neuroscience see Table 1 from the paper.
2. Manipulate your cell of interest: Cell-type specific expression of molecular tools and sensors
Combinations of classical genetic tools, for example Cre/loxP or Flp/FRT, with AAV vectors are common approaches to control transgene expression by restricting expression to genetically defined cell types. Specific transgene expression can be also achieved in nontransgenic animals by using cis regulatory elements such as promoters and enhancers, multicistronic vectors, and post-translational regulatory elements. Examples of these approaches are given in Tables 3-7.
3. Making use of AAV’s selectivity for neurons: targeting defined neuronal populations using viral strategies
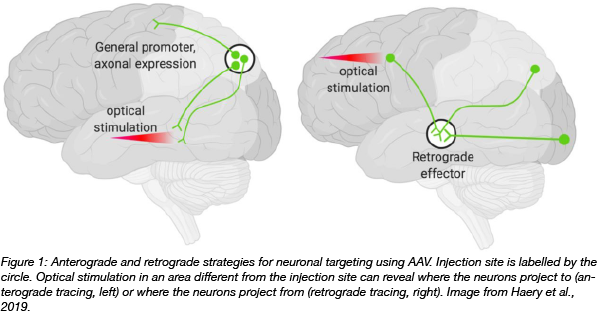
Combining different approaches can increase the specificity of cell targeting even further. For example, injecting AAVs at one location can result in expression of an optogenetic payload in neurons that project to different regions of the brain. A more locally defined set of neurons can then be targeted with optical stimuli. The restricted light exposure to a specific area in combination with delivery of AAV at the injection site results in increased specificity of the targeted cell population.
Combinatorial strategies can also be used for retrograde tracing: visualizing neurons that received information from a region. In the example shown in Figure 1, injected retrograde AAVs at one location result in opsin expression in their cell bodies. Optical stimulation in a different brain area can then reveal the cells bodies of interest.
The toolbox for neuroscience is becoming more and more versatile. To complement the wealth of genetically encoded tools available, researchers are also developing ways to improve delivery by increasing transgene cargo capacity, enhancing anterograde transport efficiency, and engineering viral capsids that target specific cell types in the brain.
No matter what experimental approach you choose, it’s essential to consider the technical limitations of each approach and to follow best practices and guidelines. It was wonderful to see the development of this practical guide by the workshop participants - a testament to openness and sharing in science.
We’re excited to support the neuroscience community by sharing not only reagents, but also method information. To complement these efforts, we’re beginning to share data through our recently launched AAV Data Hub, an open platform where researchers using AAV from our viral service can share experimental design and data. With these different approaches to open science, we aim to accelerate the rate by which sharing speeds science.
Check out the precise neuronal targeting guide here!
Additional resources on the Addgene blog
- Download the viral vectors 101 eBook
- Read our viral vector related blog posts
- Learn more about retrograde AAVs
Resources on Addgene.org
- Read our AAV guide
- Learn more about our viral service
- Find retrograde AAVs at Addgene
Topics: Viral Vectors, Viral Vector Protocols and Tips, AAV






Leave a Comment