Adenoviral vectors (AdV) are attractive vectors for research applications and gene therapy: they can be produced at high titers, can accommodate large transgenes, transduce quiescent and dividing cells, and do not integrate into the host’s genome. The main challenge with using AdV is that it triggers a strong immune response after in vivo administration, which results in the death of transduced cells and loss of transgene expression (Interestingly, the strong immunogenicity of AdVs is what makes them ideal candidates for applications in oncolysis and vaccination!)
In the 65 years since its discovery, researchers have done significant work to improve the Adv genome’s utility in research and its therapeutic potential.
Note: constructs discussed are based on Adenovirus type 5.
Three generations of adenoviral vectors
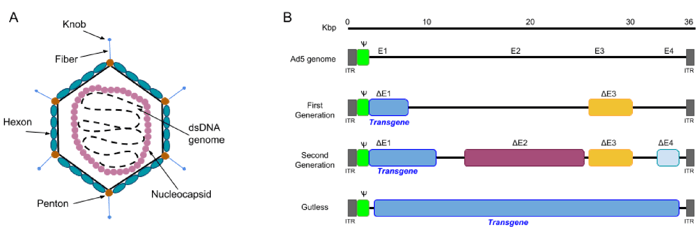
The adenovirus virion is a non-enveloped particle of ~100 nm, consisting of a capsid surrounding an inner core which contains the adenoviral genome. The genome itself is a linear double-stranded DNA of ~36kb, with inverted terminal repeats (ITR) present at both ends.
 |
|
Figure 1: A) The structure of an adenovirus: the virion is ~ 100nm in size, and consists of an icosahedral capsid made of 3 types of proteins: fiber, penton and hexon proteins. The viral genome DNA is further protected by histone-like proteins forming the nucleocapsid. B) Three generations of adenoviral vectors: the first generation vectors are deficient in the E1 and E3 regions and can accommodate up to 8.2 kb of transgene DNA. The second generation vectors are deficient in E1, E2, E3, and E4. Gutless Ad vectors (helper-dependent (HD-Ad), or high-capacity (HC-Ad__ are devoid of viral genes and therefore can accommodate up to 36 kb. Gutless Ad maintain only the ITRs and the packaging signal (Ψ), both of which are essential for assembly of the virion. Adapted from Lee et al., Adenovirus-mediated gene delivery: Potential applications for gene and cell-based therapies in the new era of personalized medicine (2017). |
1st Gen AdVs are stripped of regulatory genes E1 and E3. Without these genes, AdVs cannot replicate on their own but can be produced in E1-containing mammalian cell lines such as HEK293 cells. 1st generation AdV cloning capacity is limited to 8.2 kb, and in vivo transgene expression ceases relatively quickly due to immune response against AdV. Importantly, recombination-competent adenovirus (RCA) can be generated if the E1 gene from the packaging cell line is transferred into the AdV by recombination. It is therefore recommended to test for the presence of RCA in the viral stock by doing a plaque formation assay on A549 cells . Alternatively, antibody-based assays are commercially available for faster detection of RCA.
2nd Gen AdVs were designed to have increased cloning capacity, decreased potential for the generation of RCA and reduced immunogenicity. In addition to E1/E3, non-structural genes E2 and E4 are deleted in 2nd generation AdVs, thereby increasing cloning capacity to ~12 Kb. The ever popular pAdEasy system belongs to this category.
3rd Gen AdV (Gutless or high capacity AdV) are devoid of all viral coding sequences and retain only the ITRs and packaging signal in cis. Therefore, co-infection with a helper adenovirus is required to provide the viral proteins in trans. The Gutless AdV have generated high interest for gene therapy due their increased cloning capacity (up to 36 kb!), long-term transgene expression and negligible toxicity. However, their production is more complex and the presence of helper virus contaminants have slowed the use of this system. New and improved systems are still being developed. A detailed protocol for production of Gutless AdV can be found in reference 3.
Production, amplification, and quality control of 2nd generation AdV
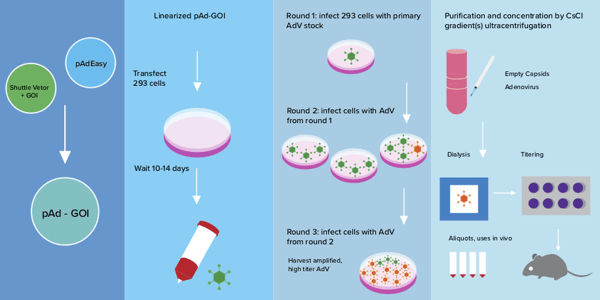
AdV Production in HEK293 cells can be broken down into 5 key steps:
- Construction of the rAdV plasmid (~1 week): The AdEasyTM system is the most popular method for creating adenoviral vector constructs. It consists of 2 plasmids: a shuttle vector (in which the transgene of interest is cloned) and pAdEasy™ which contains the adenoviral genes necessary for virus production. For detailed instructions on how to generate the final recombinant adenoviral plasmid construct, please see Addgene’s Adenoviral Guide webpage, and references 1, 2.
 *Pro-Tip*: once the correct recombinant pAdV plasmid is identified, re-transform into a bacterial strain not prone to recombination for amplification. The final purified plasmid stock should be analyzed by endonuclease restriction digest or fully sequenced to confirm its integrity.
*Pro-Tip*: once the correct recombinant pAdV plasmid is identified, re-transform into a bacterial strain not prone to recombination for amplification. The final purified plasmid stock should be analyzed by endonuclease restriction digest or fully sequenced to confirm its integrity. - Initial production (2-3 weeks) - Here you’ll produce the primary recombinant adenoviral stock (rAdV-S). HEK293 cells are transfected with the AdV plasmid construct from step #1 and allowed to stay in culture for up to 20 additional days at which time the cells are scraped, and lysed by multiple freeze-thaw cycles. The resultant lysate is your primary low titer rAdV-S, and can be stored at -80C for later use or used immediately for amplification.
 *Pro-Tip*: The cells will become completely confluent and the media will turn yellow. Do NOT change the media (add 2-3 mL of fresh media once a week), and do NOT harvest the cells before at least 10 days of incubation as it will result in very low titer.
*Pro-Tip*: The cells will become completely confluent and the media will turn yellow. Do NOT change the media (add 2-3 mL of fresh media once a week), and do NOT harvest the cells before at least 10 days of incubation as it will result in very low titer. - Amplification (1-2 weeks) - The rAdV-S is used to infect more HEK293 cells and produce more rAdV to reach higher titers. Successfully infected cells will become round and clump together ~3 days post infection. The amplified virus can be harvested once roughly 50% of the infected cells show this “cytopathic effect.” Amplification can be repeated multiple times (2-4 rounds) at increasing scale over the course of 1-2 weeks. Each round of amplification should result in a 10-100-fold increase in virus.
- Purification (2 days) - Purification is required if rAdV is to be used in vivo. The standard method for purification of rAdV uses cesium chloride (CsCl) density gradients combined with ultracentrifugation to separate rAdV from other cellular debris. Purified high-titer rAdV is then dialyzed and stored at -80C. Purification and concentration kits that do not use CsCl are commercially available.
- Titer calculation (1 or 10 days)
- Physical titer (particle count, rAdV genomes/mL) can be measured by:
- Optical density: particle concentration is measured by looking at absorbance at 260nm using UV/Vis spectrophotometry. An absorbance of at 260nm corresponds to 1.1 x 1012 particles/mL.
- To calculate the particle number: OD260 reading x dilution factor x 1.1 x 1012 particles = number of particles per mL of sample.
- The purity of the sample can be estimated by looking at the ration of DNA (260nm) versus protein (280nm) absorbance. The ratio for the purified virus should be ~1.3
- Quantitative PCR: the number of viral DNA packaged in virions is determined by a standard curve of known quantity and primers specific for a viral DNA sequence.
 *Pro-Tip*: Ad5 reference material should be used as an internal control (ATCC#VR-1516) until the assay is validated.
*Pro-Tip*: Ad5 reference material should be used as an internal control (ATCC#VR-1516) until the assay is validated. - Infectious titer: (Plaque Forming Units (PFU) or Infectious Units (IU), per mL)
- Plaque formation assay: permissive cells are infected with serial dilutions of rAdV, fixed, and then stained 10 days later. Plaques are counted and titer is calculated in terms of plaque forming units (pfu), taking initial vector dilution into account.
- Physical titer (particle count, rAdV genomes/mL) can be measured by:
You're now ready to use your purified, high-titer rAdV in your experiment. Enjoy!
References
1. Luo, Jinyong, et al. "A protocol for rapid generation of recombinant adenoviruses using the AdEasy system." Nature protocols 2.5 (2007): 1236. PubMed PMID: 17546019.
2. He TC. et al. A practical guide for using the AdEasy System.
3. Jager, Lorenz, et al. "A rapid protocol for construction and production of high-capacity adenoviral vectors." Nature protocols 4.4 (2009): 547. PubMed PMID: 19373227.
4. Mittereder, Nanette, Keith L. March, and Bruce C. Trapnell. "Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy." Journal of virology 70.11 (1996): 7498-7509. PubMed PMID: 8892868. PubMed Central PMCID: PMC190817.
5. Lee, Cody S., et al. "Adenovirus-mediated gene delivery: Potential applications for gene and cell-based therapies in the new era of personalized medicine." Genes & diseases 4.2 (2017): 43-63. PubMed PMID: 28944281. PubMed Central PMCID: PMC5609467.
Additional Resources on the Addgene Blog
- Using virus in your research - a primer for beginners
- Adenoviral delivery of CRISPR/Cas9
- 5 tips for troubleshooting viral transductions
Resources on Addgene.org
Topics: Viral Vectors, Viral Vector Protocols and Tips, Adenoviral Vectors







Leave a Comment