Lentiviruses are a powerful laboratory tool often employed to establish cell lines that stably express a gene of interest. While the general approach for using lentivirus, infect and select, seems simple, in actuality, many find using lentivirus to be time consuming, difficult, and lacking in reproducibility. Read on for some tips for getting the most out of your lentiviral transduction experiments.
Love your cells and they'll love you back
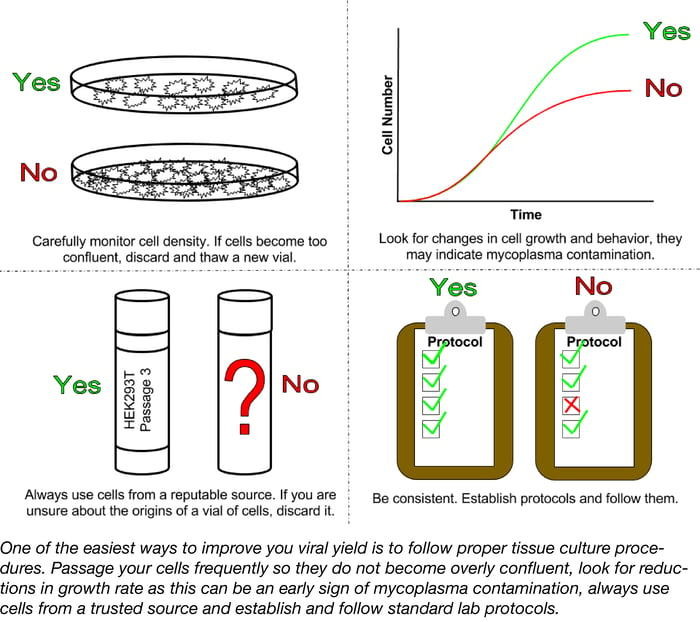
A successful lentiviral infection begins with your cells, both your packaging cells and your target cells. Poor tissue culture practices can lead to low titers and poor transductions. Cell lines should be routinely subcultured at densities that are low enough to prevent confluency but high enough to promote cell growth. Overgrown cultures stimulate quiescence, the resting phase of the cell cycle; quiescent cells do not take up nucleic acid or express transgenes as well as actively dividing cells resulting in lower lentiviral titers.
Another factor to consider is the age of your culture post thaw. In general, the higher the passage number of a culture the lower the transfection and transduction efficiencies. Cultures should only be passaged 20-30 times before discarding and thawing a fresh vial. In many labs, the history of the cells in storage may be a bit dubious due to lack of documentation and high turn-over of lab personnel. If you are unsure of the background of the lines you are using, it is best to start from scratch. Obtain a vial of the desired cell line from a reputable distributor such as the American Type Culture Collection and freeze as many early passage vials as possible.
One problem that often plagues labs and hinders experiments is mycoplasma contamination. Mycoplasma contamination has been shown to arise from a variety of sources such as serum, other cell lines, or infected personnel and can persist undetected; unlike infections with larger microbes such as yeast, fungi, or bacteria, mycoplasma can be extremely hard to detect with levels reaching 108 cells per ml before the media becomes cloudy. Mycoplasma compete with host cells for nutrients and can alter expression of receptors, ion channels, and growth factors resulting in changes to the cell line’s growth and behavior. Signs of a mycoplasma contamination can be subtle and may include reduced saturation density, decreased rates of growth, or clumping. To ensure the best possible results for any tissue culture-based experiment, labs should routinely test for mycoplasma contamination. There are several commercially available mycoplasma detection kits that use detector cell lines, ELISA, fluorometry, or PCR-based approaches to detect even low levels of contamination. For those labs that prefer a more economical mode of detection, Uphoff et. al. outline a do it yourself PCR-based approach complete with freely available positive controls.
Routinely test your cell line for mycoplasma and discard it if the culture has become overly confluent or too old. Ensuring that you are working with healthy, happy cells is one of the easiest ways to improve both viral production and infection.
Great transduction start with great virus
If having happy cells is the first step of a successful lentiviral infection, happy virus is the second. When harvesting lentivirus, some labs prefer to collect and pool the harvests from multiple time points while others opt for a single harvest after transfecting with the transfer and packaging plasmids. A benefit of the multiple harvest strategy is that each day you are supplying your producer cells with fresh medium. Daily medium exchanges help alleviate cellular stresses associated with pH changes and resource consumption due to the high cell density of the culture. In addition to benefitting your cells, medium exchanges may also impact the quality of the lentiviral particles you harvest. Studies have demonstrated that extreme changes in osmotic pressure and pH can destabilize the viral envelope. One drawback of the multiple harvest approach is that it tends to result in a higher volume of lower titer virus. In such cases you may want to consider concentrating your viral prep.
Storage can also play a role in the success of your lentiviral transduction. The effect of freeze-thaw cycles on lentiviral stability depends on a variety of factors such as the transgene expressed, the viral storage buffers used, and other experimental parameters. For example, while freeze thaw is generally not recommended, Holic et al. found that lentivirus produced at pH 6 are resistant to multiple freeze thaw cycles. Consequently, it is best to avoid freeze-thawing lentiviral preps unless absolutely necessary. Many labs try to use freshly collected lentivirus for downstream applications. While this ensures that the highest titer virus is used, it is not always feasible, especially when the lentiviral stock needs to be titrated. If storing for less than a day, lentivirus can be kept at 4°C. For long-term storage, viral preps should be divided into single-use aliquots, and stored at -80°C. Some reports suggest rapidly freezing virus in a dry ice/ethanol bath or liquid nitrogen prior to storing.
Know your target cells
Labs tend to follow the same general procedure for lentiviral infections, produce the virus, titer the virus (read this blog post for tips on titering your lentiviral prep!) and infect. While this approach is a great start to a successful infection, one point to consider is how the virus is being titered. Most titering approaches transduce into highly permissive cell lines such as HEK293, H0 or A549 and assume that the results obtained in these lines can be translated to other lines. Differences in the physiology of different cell types, however, can make this assumption incorrect. One of the earliest steps in lentiviral transduction is the interaction between the viral envelop and the receptors on the target cell surface. The possible interactions and hence possible targets for a lentivirus are determined by the envelop protein. Many times lentiviral vectors are engineered with non-native envelopes, a process called pseudotyping. Lentiviruses pseudotyped with the VSV-G envelope protein use the LDL receptor to infect cells and have a broad host range. However, viruses pseudotyped with other envelope proteins may have a limited number of targets and therefore titers obtained in one cell type may not translate well to other cell types. To overcome this issue, try transducing with a range of dilutions of your lentiviral prep. Hopefully, by using more concentrated dilutions you will be able to overcome differences in receptor expression.
It's all in the mix
Even before the lentiviral envelope protein attaches to cell surface receptors, other important binding events take place during the course of infection. These binding events can be impaired by the electrostatic repulsion of the negatively charged cell with the virus. To circumvent this problem, transductions typically employ the positively charged polycation, polybrene. Interestingly, one study found that alternative polycations such as DEAE-dextran can improve transduction efficiency. In addition, this study found that transduction efficiency varied not only between serum from different manufacturers but also between different lots of serum from the same source. Labs that routinely produce lentivirus may want to consider testing a variety of cation and serum sources to ensure the best possible transduction.
Be consistent
Once your lab establishes the best conditions for specific applications, stick with them! Changes in such variables as cell density, volume, incubation time, and MOI can all impact the success of your lentiviral infection. To ensure high quality and reproducible results always care for your cell lines and virus properly and be consistent when following protocols.
Need Virus? Check out Addgene's New Viral Service!
References
1. Hay, R.J., Macy, M.L. & Chen, T.R. “Mycoplasma infection of cultured cells.” Nature 339 (1989). 487–488. PubMed PMID: 2725683.
2. Miller, C.J., Kassem H.S., Pepper S.D., Hey Y., Ward T.H., Margison G.P. “Mycoplasma infection significantly alters microarray gene expression profiles.” Biotechniques 35.4 (2003). 812-4. Pubmed PMID: 14579747.
3. Drexler, H.G., Uphoff, C.C. “Mycoplasma contamination of cell cultures: Incidence, sources, effects, detection, elimination, prevention.” Cytotechnology 39.2 (2002). 75-90. Pubmed PMID: 19003295.
4. Uphoff, C.C., Drexler, H.G. “Detection of mycoplasma contaminations.” Methods in Molecular Biology 946 (2013). 1-13. Pubmed PMID: 23179822.
5. Segura, M.M., Kamen, A., Garnier, A. “Downstream processing of oncoretroviral and lentiviral gene therapy vectors.” Biotechnology Advances 24.3 (2006). 321-37. Pubmed PMID: 16448798.
6. Kutner, R.H., Zhang, X.Y., Reiser, J.. "Production, concentration and titration of pseudotyped HIV-1-based lentiviral vectors."Nature protocols 4.4 (2009): 495-505. PubMed PMID: 19300443.
7. Holic, N., Seye, A.K., Majdoul, S., Martin, S., Merten, O.W., Galy, A., Fenard, D. ”Influence of mildly acidic pH conditions on the production of lentiviral and retroviral vectors.” Human Gene Therapy Clinical Development 25.3 (2014). 175-85. Pubmed PMID: 25073060.
8. Finkelshtein, D., Werman, A., Novick, D., Barak, S., Rubinstein, M. “LDL receptor and its family members serve as the cellular receptors for vesicular stomatitis virus.” Proceedings of the National Academy of Sciences 110.18 (2013). 7306-11. Pubmed PMID: 23589850.
9. Cronin, J., Zhang, X.Y., Reiser, J. “Altering the tropism of lentiviral vectors through pseudotyping.” Current Gene Therapy 5.4 (2005). 387-98. Pubmed PMID: 16101513. PubMed Central PMCID: PMC1368960.
10. Denning, W., Das, S., Guo, S., Xu, J., Kappes, J.C., Hel, Z. “Optimization of the transductional efficiency of lentiviral vectors: effect of sera and polycations.” Molecular Biotechnology 53.3 (2013). 308-14. Pubmed PMID: 22407723. PubMed Central PMCID: PMC3456965.
11. Zhang B., Metharom P., Jullie H., Ellem K.A., Cleghorn G., West M.J., Wei MQ. “The significance of controlled conditions in lentiviral vector titration and in the use of multiplicity of infection (MOI) for predicting gene transfer events.” Genetic Vaccines and Therapy 2.1 (2004) Pubmed PMID: 15291957. PubMed Central PMCID: PMC514534.
Additional Resources on the Addgene Blog
- Get Tips for Titering Your Lentivirus
- Learn All About Viral Vector Elements
- Learn How to Do Genome-Wide Screens with CRISPR Lentiviral Vectors
Additional Resources on Addgene.org
- Find Viral Vectors for Your Research
- Brush Up on All Things Lentivirus with our Lentiviral Guide Pages
- Deposit Your Lentiviral Vectors
Topics: Viral Vectors, Viral Vector Protocols and Tips, Retroviral and Lentiviral Vectors






Leave a Comment